A typical battery, such as a AA or C battery has a case or container. Molded to the inside of the case is a cathode mix, which is ground manganese dioxide and conductors carrying a naturally-occurring electrical charge. A separator comes next. This paper keeps the cathode from coming into contact with the anode, which carries the negative charge. The anode and the electrolyte (potassium hydroxide) are inside each battery. A pin, typically made of brass, forms the negative current collector and is in the center of the battery case.
Each battery has a cell that contains three components: two electrodes and an electrolyte between them. The electrolyte is a potassium hydroxide solution in water. The electrolyte is the medium for the movement of ions within the cell and carries the iconic current inside the battery.
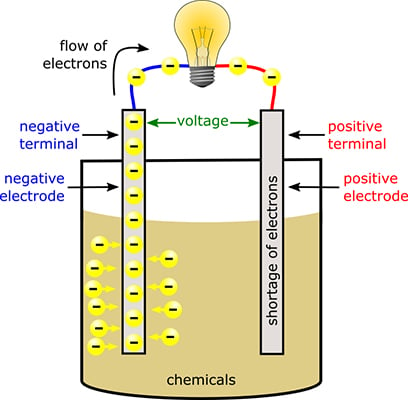
The positive and negative terminals of a battery are connected to two different types of metal plates, known as electrodes, which are immersed in chemicals inside the battery. The chemicals react with the metals, causing excess electrons to build up on the negative electrode (the metal plate connected to the negative battery terminal) and producing a shortage of electrons on the positive electrode (the metal plate connected to the positive battery terminal).
Flashlight or smaller batteries, usually labeled A, AA, C, or D have the terminals built into the ends of the batteries. That's why the battery compartment of your flashlight has a + and a - sign, making it easier for you to install your batteries the correct direction. Larger batteries, like those in a car, have terminals that extend out from the battery. (They generally look like large screw tops.)
The difference in the number of electrons between the positive and negative terminals creates the force known as voltage. This force wants to even out the teams, so to speak, by pushing the excess electrons from the negative electrode to the positive electrode. But the chemicals inside the battery act like a roadblock and prevent the electrons from traveling between the electrodes. If there's an alternate path that allows the electrons to travel freely from the negative electrode to the positive electrode, the force (voltage) will succeed in pushing the electrons along that path.
When you connect a battery to a circuit, you provide that alternate path for the electrons to follow. So the excess electrons flow out of the battery via the negative terminal, through the circuit, and back into the battery via the positive terminal. That flow of electrons is the electric current that delivers energy to your circuit.
When the electrodes are connected via a circuit, for example, the terminals inside a flashlight or those in your vehicle, the chemicals in the electrolyte start reacting.
As electrons flow through a circuit, the chemicals inside the battery continue to react with the metals, excess electrons keep building up on the negative electrode, and electrons keep flowing to try to even things up — as long as there's a complete path for the current. If you keep the battery connected in a circuit for a long time, eventually all the chemicals inside the battery are used up and the battery dies (it no longer supplies electrical energy).
The electrolyte oxides the anode's powered zinc. The cathode's manganese dioxide/carbon mix reacts with the oxidized zinc to produce electricity. Interaction between the zinc and the electrolyte produces gradually slow the cell's action and lowers its voltage.
The collector is a brass pin in the middle of the cell that conducts electricity to the outside circuit.
Note that the two electrodes in every battery are made from two different materials, both of which must be electrical conductors. One of the materials gives electrons and the other receives them, which makes the current flow.

