An ion example
For example, in the compound sodium chloride — table salt — the sodium atom has a positive charge and the chlorine atom has a negative charge.The neutral sodium atom has 11 protons and 11 electrons, which means it has 11 positive charges and 11 negative charges. Overall, the sodium atom is neutral, and it’s represented like this: Na. But the sodium ion contains one more positive charge than negative charge, so it’s represented like this:
This unequal number of negative and positive charges can occur in one of two ways: An atom can gain a proton (a positive charge) or lose an electron (a negative charge).
Cations and anions
So which process is more likely to occur? In general, it’s easy to gain or lose electrons but very difficult to gain or lose protons. So atoms become ions by gaining or losing electrons. And ions that have a positive charge are called cations.The progression goes like this: The sodium ion shown above is formed from the loss of one electron. Because it lost an electron, it has more protons than electrons, or more positive charges than negative charges, which means it’s now called the:
Likewise, when the neutral magnesium atom loses two electrons, it forms the:
Now consider the chlorine atom in sodium chloride. The neutral chlorine atom has acquired a negative charge by gaining an electron. Because it has unequal numbers of protons and electrons, it’s now an ion. And because ions that have a negative charge are called anions, it’s now called the:
Other details about ions
Here are some extra tidbits about ions:-
You can write electron configurations and energy level diagrams for ions. The neutral sodium atom (11 protons) has an electron configuration of:
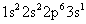
The sodium cation has lost an electron — the valence electron, which is farthest away from the nucleus (the 3s electron, in this case). The electron configuration of the sodium ion is:
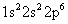
-
The electron configuration of the chloride ion is:
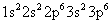
This is the same electron configuration as the neutral Argon atom. If two chemical species have the same electron configuration, they’re said to be isoelectronic.
-
The preceding examples are all monoatomic (one atom) ions. But polyatomic (many atom) ions do exist. The ammonium ion is a polyatomic ion, or, specifically, a polyatomic cation. It is written as:
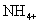
The nitrate ion, is also a polyatomic ion, or, specifically, a polyatomic anion. It is written as
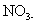
-
Ions are commonly found in a class of compounds called salts, or ionic solids. Salts, when melted or dissolved in water, yield solutions that conduct electricity.
A substance that conducts electricity when melted or dissolved in water is called an electrolyte. Table salt — sodium chloride — is a good example.
On the other hand, when table sugar (sucrose) is dissolved in water, it becomes a solution that doesn’t conduct electricity. So sucrose is a nonelectrolyte.
Whether a substance is an electrolyte or a nonelectrolyte gives clues to the type of bonding in the compound. If the substance is an electrolyte, the compound is probably ionically bonded. If it’s a nonelectrolyte, it’s probably covalently bonded.

