When four nonidentical groups are attached to a double bond in an alkene, you must use the E/Z system of nomenclature to assign the stereochemistry of the double bond. (E stands for the German word entgegen, which means "opposite," while Z stands for the German word zusammen, which means "together.")
To use the E/Z system of nomenclature, you have to play a game of high-low. First, you must decide which substituent on each carbon is given higher priority and which is given lower priority using the Cahn–Ingold–Prelog prioritizing scheme on both sides of the alkene. This figure helps you visualize the process.
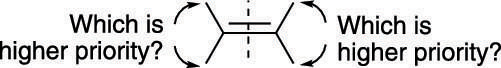
If the two high-priority substituents are on the same side of the double bond, the alkene is given the Z nomenclature; if the highs are on opposite sides of the double bond, the alkene is given the E nomenclature, as shown here.

Here's how to prioritize the double-bond substituents using the Cahn–Ingold–Prelog prioritizing scheme.
Individual substituents: The substituent whose first atom has the highest atomic number gets the highest priority. For example, iodine would be higher priority than bromine, which would be higher than fluorine.
Ties: In the case of a tie (both first atoms are carbon, for example), proceed to the next atom and decide which atom has the higher atomic number. If you get another tie, keep going until the tie is broken, as shown here.
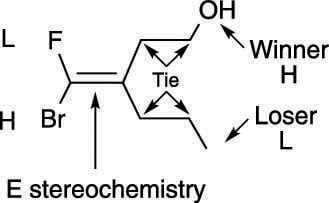 Determining the priorities of double-bond substituents in cases of ties.
Determining the priorities of double-bond substituents in cases of ties.Multiple bonds: You treat multiple bonds in a somewhat strange fashion using the Cahn–Ingold–Prelog prioritizing scheme. Using this prioritizing scheme, you treat multiple bonds as if they were multiple single bonds. A carbon double-bonded to oxygen, for example, would be treated as if the carbon had two carbon-oxygen single bonds (and as if each oxygen were bonded back to carbon). Which is higher priority, the carbon double-bonded to oxygen or the carbon triple-bonded to nitrogen, as shown here?
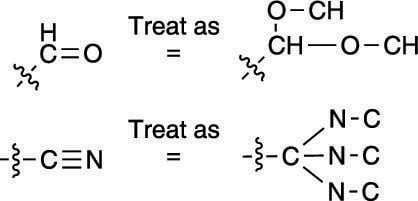 Treating multiple bonds using the Cahn–Ingold–Prelog prioritizing scheme.
Treating multiple bonds using the Cahn–Ingold–Prelog prioritizing scheme.The carbon double-bonded to oxygen is higher priority because oxygen has a higher atomic number than nitrogen. (The fact that one group has three C-N bonds and the other has only two C-O bonds is irrelevant; the number of bonds only matters when comparing identical bonds, so C=NH would be higher priority than C-NH2.)

