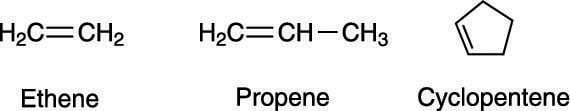
Whereas the names of alkanes end with the suffix –ane, alkenes end with the suffix –ene. A two-carbon alkene, therefore, is named ethene; a three-carbon alkene is named propene, and an alkene in a five-membered ring is named cyclopentene, as shown here.
For molecules in which the double bond can be put in more than one position, you must put a number in front of the parent name to indicate the position of the double bond. For example, two alkene locations in pentene are possible, as shown here, so a number must be placed in front of the name to show the location of the double bond.

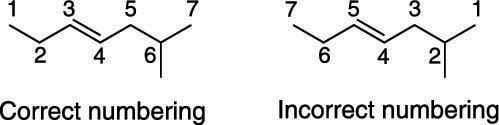
The parent chain is numbered so that the alkene is given the lowest possible number. For example, you wouldn't name the pentene 3-pentene because that would be an identical structure to 2-pentene.
The next figure shows the correct and incorrect numbering in a seven-carbon chain with a methyl substituent. Notice that giving the smaller number to the double bond takes precedence over assigning the smaller number to the substituent.
Additionally, the alkene must be included in the parent chain, even if a longer chain of carbon atoms can be found that doesn't include the alkene. The next figure shows how this works.

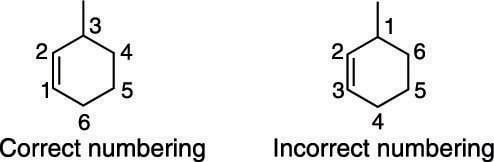
To number an alkene in a ring, number around the ring so that the double bond has the lowest possible number, as shown here.
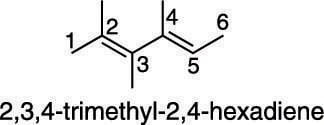
The naming of alkenes with more than one double bond requires the use of a prefix (such as di–, tri–, tetra–, and so forth) to indicate the number of double bonds in the molecule. Also, the position of all double bonds is indicated with numbers at the beginning of the name. An example of how this is done is shown here.
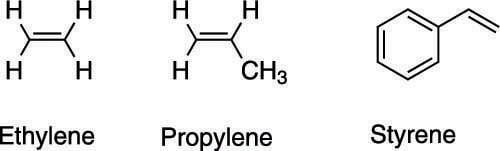
Although alkenes can be named systematically using the IUPAC nomenclature (the official nomenclature system of chemists), some alkenes have common names that come from the olden days. These older names (some of which are shown here) are still often seen in the literature and need to be memorized.
For some smaller alkenes, the suffix –ylene is used instead of simply –ene. Ethene, for example, is usually called ethylene, and propene is called propylene. Styrene — the compound used to make the plastic polystyrene — is the common name for a molecule that consists of a double bond jutting off a benzene ring.
How to identify stereoisomers of an alkene
Alkenes, which are molecules containing carbon-carbon double bonds, have the possibility of having stereoisomers, just as ring systems do. This is because, unlike carbon-carbon single bonds, which are free to rotate, double bonds are fixed and rigid. In other words, rotation around carbon-carbon double bonds is not possible at reasonable temperatures.Molecules that have the same connectivity of atoms, but different orientations of those atoms in space are called stereoisomers.
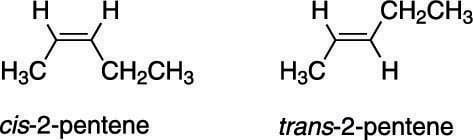
In general, when two identical groups are on the same side of the double bond, the molecule is said to possess cis stereochemistry; when two identical groups are on opposite sides of the double bond, the molecule is said to possess trans stereochemistry.
You can only have cis-trans stereochemistry in rings and on double bonds. You can't have cis-trans isomers on single bonds due to the rapid free rotation of these bonds at room temperature.

