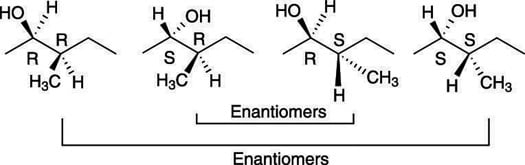
When more than one chiral center is present in a molecule, you have the possibility of having stereoisomers that are not mirror images of each other. Such stereoisomers that are not mirror images are called diastereomers.
Typically, you can only have diastereomers when the molecule has two or more chiral centers.
The maximum number of possible stereoisomers that a molecule can have is a function of 2n, where n is the number of chiral centers in the molecule. Therefore, a molecule with five chiral centers can have up to 25 or 32 possible stereoisomers! As the number of chiral centers increases, the number of possible stereoisomers for that compound increases rapidly.
For example, the molecule shown here has two chiral centers.
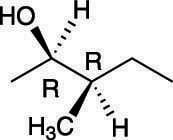
Because this molecule has two chiral centers, it can have a total of 22, or 4, possible stereoisomers, of which only one will be the enantiomer of the original molecule.
Enantiomers are stereoisomers that are mirror images of each other.
Because both chiral centers in this molecule are of R configuration, the enantiomer of this molecule would have the S configuration for both chiral centers. All the stereoisomers of this molecule are shown in the next figure. Those molecules that are not enantiomers of each other are diastereomers of each other.