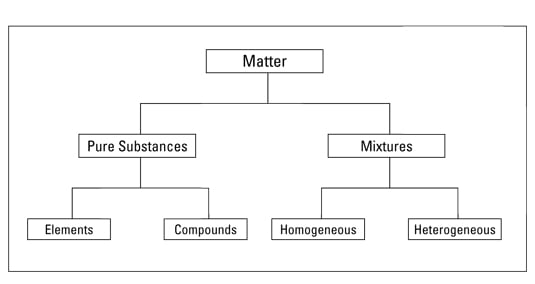
Pure substances
A pure substance has a definite and constant composition — like salt or sugar. A pure substance can be either an element or a compound, but the composition of a pure substance doesn’t vary.Elements
An element is composed of a single kind of atom. An atom is the smallest particle of an element that still has all the properties of the element.Here’s an example: Gold is an element. If you slice and slice a chunk of gold until only one tiny particle is left that can’t be chopped any more without losing the properties that make gold gold, then you’ve got an atom.
The atoms in an element all have the same number of protons. Protons are subatomic particles — particles of an atom. Elements are the building blocks of matter. And they’re represented in a strange table you may have seen at one time or another — the periodic table.
Compounds
A compound is composed of two or more elements in a specific ratio. For example, water is a compound made up of two elements, hydrogen (H) and oxygen (O). These elements are combined in a very specific way — in a ratio of two hydrogen atoms to one oxygen atom, known as:Many compounds contain hydrogen and oxygen, but only one has that special 2 to 1 ratio we call water. The compound water has physical and chemical properties different from both hydrogen and oxygen — water’s properties are a unique combination of the two elements.
Chemists can’t easily separate the components of a compound. They have to resort to some type of chemical reaction.
Mixtures
Mixtures are physical combinations of pure substances that have no definite or constant composition — the composition of a mixture varies according to who prepares the mixture.Although chemists have a difficult time separating compounds into their specific elements, the different parts of a mixture can be easily separated by physical means, such as filtration.
For example, suppose you have a mixture of salt and sand, and you want to purify the sand by removing the salt. You can do this by adding water, dissolving the salt, and then filtering the mixture. You then end up with pure sand.
Mixtures can be either homogeneous or heterogeneous:
-
A homogeneous mixture, sometimes called a solution, is relatively uniform in composition; every portion of the mixture is like every other portion.
For example, if you dissolve sugar in water and mix it really well, your mixture is basically the same no matter where you sample it.
-
A heterogeneous mixture is a mixture whose composition varies from position to position within the sample.
For example, if you put some sugar in a jar, add some sand, and then give the jar a couple of shakes, your mixture doesn’t have the same composition throughout the jar. Because the sand is heavier, there’s probably more sand at the bottom of the jar and more sugar at the top.

