Air pollution has the ability to affect the earth’s entire ecosystem, as environmental scientists realized when they found direct links between ozone layer destruction and manmade pollutants called CFCs. To understand the ozone layer, you need to be familiar with the atmosphere as a whole. Earth is surrounded by layers of gas with different temperatures, pressures, and compositions.
This figure illustrates each of the layers in earth’s atmosphere.
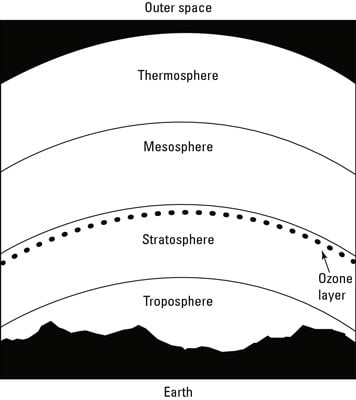
Starting at ground level, the layers proceed up as follows:
Troposphere
Stratosphere
Mesosphere
Thermosphere
So where’s the ozone layer in this list? Actually, the ozone layer isn’t a specific level of the atmosphere; rather, it’s a thick band of ozone molecules at the top of the stratosphere level.
How is the ozone layer created?
The earth’s ozone layer is composed of molecules of ozone, or O3. These molecules provide a protective layer around the earth, absorbing a large portion of the sun’s radiation, particularly some of the harmful ultraviolet (UV) rays that cause sunburn.
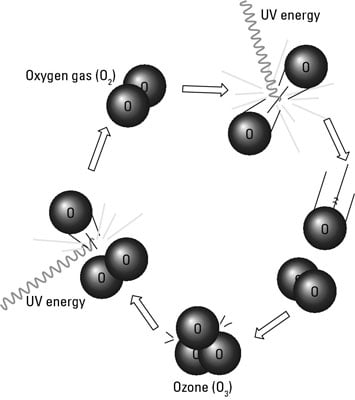
Ozone is naturally created when UV radiation hits oxygen gas molecules (O2) and breaks some of them apart into single oxygen atoms. These single atoms of oxygen then bond with other O2 gas molecules, forming O3. In a similar reaction, O3 molecules can be broken down into atoms of O and molecules of O2.
In this way, ozone, oxygen gas, and free oxygen atoms are constantly being combined, broken apart, and recombined in the stratosphere. These reactions are driven by the energy from UV radiation, which is absorbed in the process.
These formulas and figure illustrate this process:
O2 + O + UV energy → O3
O3 + UV energy → O2 + O
How is the ozone layer depleted?
In the 1980s, scientists noticed that the ozone layer over the South Pole was becoming much thinner than usual. After some study, atmospheric scientists recognized that this ozone depletion was a result of air pollutants from human activities — specifically, the release of nontoxic, aerosol gas molecules called chlorofluorocarbons.
Chlorofluorocarbons, or CFCs, are chemically inactive (inert) gas molecules commonly used in fire extinguishers, many different types of aerosol cans (such as hairspray), and refrigerator coolants beginning in the 1920s. Using such products releases CFCs into the atmosphere, an occurrence no one worried too much about for many decades; after all, the molecules are chemically inert and nontoxic, so no problem, right? Not so fast.
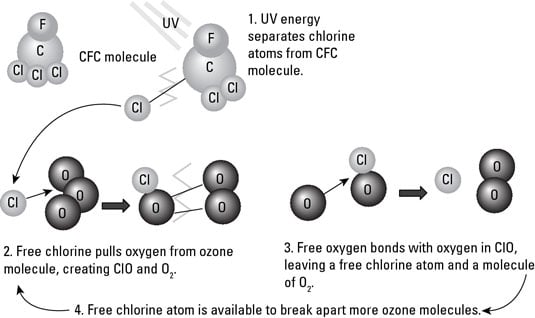
After the stable and inert CFCs are in the atmosphere, they don’t break down or react with anything around them until they reach the stratosphere, where the UV radiation is very intense. The high levels of energy in the UV radiation are strong enough to break chlorine atoms away from the CFC molecule. These free chlorine atoms then break apart ozone molecules in the following reaction:
O3 + Cl → ClO + O2
Although the third oxygen atom in the O3 molecule is bonded to the chlorine atom, it’s more attracted to other, single oxygen atoms, so after the ClO compound is created, it quickly changes again, like this:
ClO + O → Cl + O2
In these reactions, the chlorine from the CFC molecule is acting as a catalyst — it’s helping to break apart O3 molecules and forming O2 molecules. The result is that, unlike the natural cycle of ozone formation and recycling, the ozone is being broken down into O2 and not recycled back into O3 because no free oxygen atoms are left for O2 to combine with. This figure shows this process.
The presence of chlorine atoms in the atmosphere changes the natural ozone reaction (the one that absorbs UV energy) reaction from
O2 + O ↔ O3
to
O3 + O → 2O2
The second reaction is driven by the presence of chlorine atoms and doesn’t absorb UV energy. The result is a stratosphere lacking in ozone molecules; because of this change, the protective layer of ozone has gradually become thinner and less protective all over the world.

