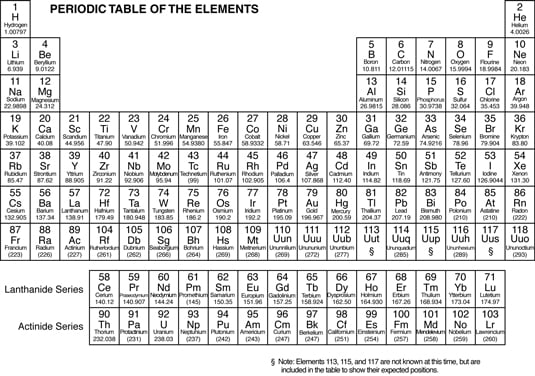
A pattern of repeating order is called periodicity. In the mid-1800s, Dmitri Mendeleev, a Russian chemist, noticed a repeating pattern of chemical properties in elements.
Mendeleev arranged the elements in order of increasing atomic mass, to form something that resembles the modern periodic table. He was even able to predict the properties of some of the then-unknown elements. Later, the elements were rearranged in order of increasing atomic number.
The periodic table enables chemists and chemistry students to:
Learn the properties of families of elements (instead of learning the properties of each element), thus saving a lot of time and effort.
Find the relationships among elements and figure out the formulas of many different compounds.
Examine the atomic numbers, mass numbers, and information about the number of valence electrons.
The elements in the following periodic table are arranged in order of increasing atomic number. The atomic number (number of protons) is located right above the element symbol.
Under the element symbol is the atomic mass, or atomic weight (sum of the protons and neutrons). Atomic mass is a weighted average of all naturally occurring isotopes.
Notice also that two rows of elements — Ce-Lu (commonly called the Lanthanides) and Th-Lr (the Actinides) — have been pulled out of the main body of the periodic table. If they were included in the main body of the periodic table, the table would be much larger.
Click here to view this table.
The periodic table is composed of horizontal rows called periods. The periods are numbered 1 through 7 on the left-hand side of the table. The vertical columns are called groups, or families. Members of these families have similar properties.
The families may be labeled at the top of the columns in one of two ways. The older method uses Roman numerals and letters. Many chemists prefer and still use this method. The newer method simply uses the numbers 1 through 18.

