Photons are one of the fundamental particles of physics that physicists hope to explain using string theory. Einstein received the Nobel Prize not for relativity, but instead for his work in using Planck’s idea of the quantum to explain another problem — the photoelectric effect.
He went further than Planck, suggesting that all electromagnetic energy was quantized. Light, Einstein said, moved not in waves, but in packets of energy. These packets of energy became called photons.
The photoelectric effect occurs when light shines on certain materials that then emit electrons. It’s almost as if the light knocks loose the electrons, causing them to fly off the material. The photoelectric effect was first observed in 1887 by Heinrich Hertz, but it continued to puzzle physicists until Einstein’s 1905 explanation.
Modern solar cells work off the same principle as the photoelectric effect. Composed of photoelectric materials, they take electromagnetic radiation in the form of sunlight and convert it into free electrons. Those free electrons then run through wires to create an electric current that can power devices such as ornamental lights in your flowerbed or NASA’s Martian rovers.
At first, the photoelectric effect didn’t seem that hard to explain. The electrons absorbed the light’s energy, which caused the electrons to fly off the metal plate. Physicists still knew very little about electrons — and virtually nothing about the atom — but this made sense.
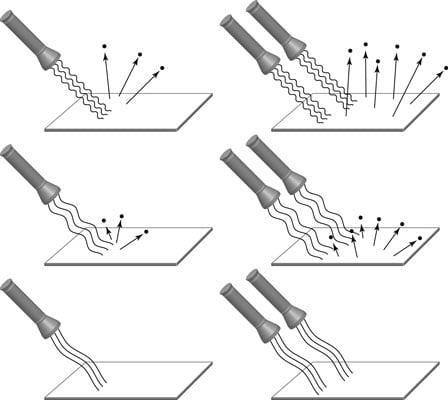
As expected, if you increased the light’s intensity (the total energy per second carried by the beam), more electrons definitely were emitted (see the top of the figure). There were two unexpected problems though:
Above a certain wavelength, no electrons are emitted — no matter how intense the light is (as shown in the bottom of the figure).
When you increase the light’s intensity, the speed of the electrons doesn’t change.
Einstein saw a connection between this first problem and the ultraviolet catastrophe faced by Max Planck, but in the opposite direction. The longer wavelength light (or light with lower frequency) failed to do things that were being achieved by the shorter wavelength light (light with higher frequency).
Planck had created a proportional relationship between energy and frequency. Einstein again did what he was best at — he took the mathematics at face value and applied it consistently. The result was that the high frequency light had higher energy photons, so it was able to transfer enough energy into the electron for it to get knocked loose.
The lower frequency photons didn’t have enough energy to help any electrons escape. The photons had to have energy above a certain threshold to knock the electrons loose.
Similarly, the second problem of the noneffect of light’s intensity on an electron’s speed is also solved by Einstein’s quantum view of light. Each photon’s energy is based on its frequency (or wavelength), so increasing the intensity doesn’t change the energy of each photon; it only increases the total number of photons.
This is why increasing the intensity causes more electrons to get emitted, but each electron maintains the same speed. The individual photon knocks out an electron with the same energy as before, but more photons are doing the same job. No single electron gets the benefit of the increase in intensity.
Based on the principle that the speed of light was constant (the basis of his special theory of relativity), Einstein knew that these photons would always move at the same velocity, c. Their energy would be proportional to the frequency of the light, based on Planck’s definitions.

