Creation and annihilation may sound like big make-or-break-the-universe kinds of ideas, but they play a starring role in the quantum world when you're working with harmonic oscillators. You use the creation and annihilation operators to solve harmonic oscillator problems because doing so is a clever way of handling the tougher Hamiltonian equation. Here's what these two operators do:
Creation operator. The creation operator raises the energy level of an eigenstate by one level, so if the harmonic oscillator is in the fourth energy level, the creation operator raises it to the fifth level.
Annihilation operator. The annihilation operator does the reverse, lowering eigenstates one level.
These operators make it easier to solve for the energy spectrum without a lot of work solving for the actual eigenstates. In other words, you can understand the whole energy spectrum by looking at the energy difference between eigenstates.
Here's how people usually solve for the energy spectrum. First, you introduce two new operators, p and q, which are dimensionless; they relate to the P (momentum) operator this way:
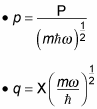
You use these two new operators, p and q, as the basis of the annihilation operator, a, and the creation operator,
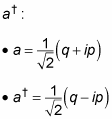
Now you can write the harmonic oscillator Hamiltonian like this, in terms of
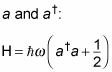
As for creating new operators here, the quantum physicists went crazy, even giving a name to
So here's how you can write the Hamiltonian:
The N operator returns the number of the energy level of the harmonic oscillator. If you denote the eigenstates of N as
you get this, where n is the number of the nth state:
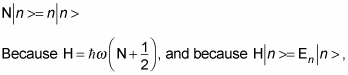
then by comparing the previous two equations, you have
Amazingly, that gives you the energy eigenvalues of the nth state of a quantum mechanical harmonic oscillator. So here are the energy states:
The ground state energy corresponds to n = 0:
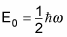
The first excited state is
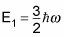
The second excited state has an energy of
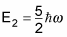
And so on. That is, the energy levels are discrete and nondegenerate (not shared by any two states). Thus, the energy spectrum is made up of equidistant bands.

