When you have identified all the fragments of a molecular compound, you can use information such as NMR peak splitting, chemical shift, and degrees of unsaturation to help identify the compound's final structure.
Often, when you're starting, the best way to go from fragments to a possible structure is to simply brainstorm all the possible molecules that could include the fragments you listed, because in many cases there will be more than one way to put all the fragments together. After you've brainstormed all the possible structures, you systematically eliminate all the incorrect structures. You can eliminate the incorrect structures based on a few different factors. Most often, the incorrect structures will not produce the peak splitting that you see in your NMR spectrum, or will have symmetry when your NMR spectrum suggests that the molecule is not symmetrical. But sometimes you may need to use the chemical shift to eliminate incorrect structures. This procedure may seem tedious, but after working a few problems, you'll develop an intuition about which structures are likely correct, and putting together the fragments to make the correct structure will take no time at all.
After working several problems, you'll notice some common patterns. A quartet that integrates for 2H and a triplet that integrates for 3H on a spectrum is often indicative of an ethyl group (–CH2CH3). Likewise, a multiplet that integrates for 1H and a doublet that integrates for 6H usually suggest an isopropyl group (–CH(CH3)2). A singlet integrating for 9H is usually a t-butyl group (–C(CH3)3).
Here's an example: On an exam, you are asked to determine the structure for the molecular formula C5H10O (you are also given the 1H NMR and IR spectra for the molecule). After identifying all of the molecule's fragments, you narrow down the possible structures to two, as shown here.
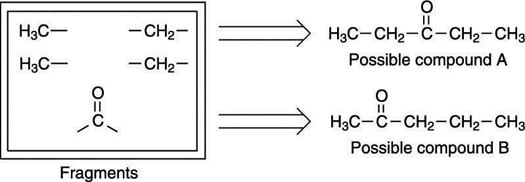
Because the carbonyl in this example is a ketone, the fragments can only be pieced together in two ways. Note that one of these ways gives a symmetric molecule (possible compound A, where the carbonyl group is in the center of the molecule) while the other way gives a molecule that's not symmetric (possible compound B, where the carbonyl group is on one side of the molecule).
Now that the choices are narrowed down to two, you want to rule out the one that is the least consistent with the peak splitting and the chemical shift. Try possible compound A first.
You can easily rule out this compound because it has a center of symmetry — that is, the left-hand side of the molecule is identical to the right-hand side. Therefore, you would expect to see just two peaks in the 1H NMR for this compound rather than the four you see in the next figure, because both methyl (CH3) groups would be in identical chemical environments, and both methylene (CH2) groups would also be in identical chemical environments.
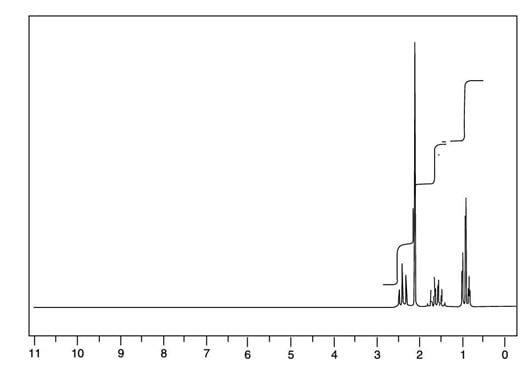
This leaves compound B as the only possible structure for the molecular formula C5H10O.

