In addition to being one of the most valuable reactions in organic chemistry, Diels–Alder reactions also tend to look very confusing. Fortunately, you can follow these four simple steps to determine the products of these reactions:
Orient the diene and the dienophile correctly.
In this step, you make sure that the double bonds are oriented correctly (the diene double bonds are pointing in the direction of the dienophile), and that the diene is in the s-cis conformation (if it isn't, you need to rotate it so that it is).
Number the diene carbons (1 through 4).
You can start the numbering on either end of the diene. The numbering is just a way for you to keep track of where bonds should be formed to make the product.
Work the reaction.
Make a bond from the number-one carbon on the diene to one side of the dienophile. Then make a bond from the number-four carbon to the other side of the dienophile. Get rid of the two double bonds in the starting material between carbons 1 and 2 and 3 and 4, and put a double bond between carbons 2 and 3 in the product.
Make sure you have the correct stereochemistry.
If you start with a cis disubstituted dienophile, the product should be substituted cis; if the dienophile starts trans, it should end up trans. With bicyclo products, make sure that you show endo stereochemistry in the product.
Now try taking the problem shown here through the four steps.
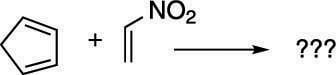
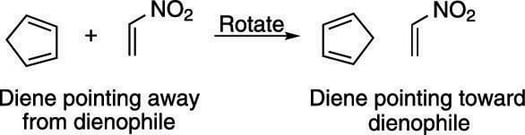
Step 1: Orienting the molecule
Start by orienting the diene so that the two double bonds in the diene point in the direction of the dienophile (with the single bond that connects the double bonds oriented away from the dienophile). In this case, you need to rotate the diene 180 degrees, as shown in the next figure. Because this diene is in a ring, you don't have to worry about twisting the diene to the s-cis conformation because dienes in rings are locked in the s-cis conformation.
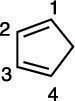
Step 2: Numbering the diene
Next, number the carbons on the diene (1 through 4). Start the numbering on any side of the diene. In the next figure, the numbering begins on the top.
Step 3: Making the bonds
Now that everything's lined up, take a deep breath and work the reaction. You make bonds between the number-one carbon on the diene and the dienophile, and between the number-four carbon and the dienophile. You end with a double bond between carbons two and three. Notice that in the reaction shown in the next figure, the fifth carbon in the diene ring is flipped up, forming a pointed "bridge" between carbons one and four.
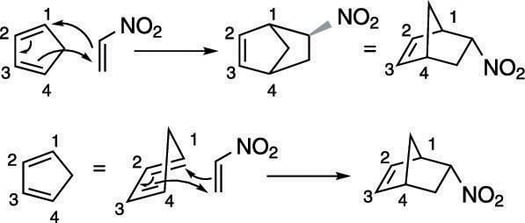
Step 4: Checking the stereochemistry
Make sure you have the correct stereochemistry. Because you have a bicyclic product, you need to make sure that the nitro substituent is endo (points down from the carbon bridge at the top of the molecule). And that's it!

