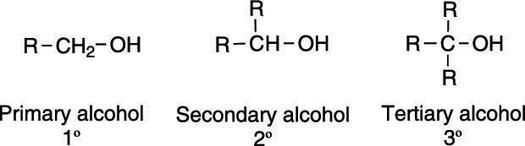
Alcohols are molecules that contain a hydroxyl (OH) group, and they're typically classified by the carbon to which the hydroxyl group is attached. If the carbon bonded to the OH is attached to one other alkyl group, the alcohol is classified as primary (1°); if the carbon is attached to two other alkyl groups, the alcohol is classified as secondary (2°); if the carbon is attached to three alkyl groups, the alcohol is classified as tertiary (3°). The three alcohol classifications are shown here.
You can name alcohols just by extending the nomenclature rules used for alkanes. To name an alcohol you follow these five steps:
-
Determine the parent name of the alcohol by looking for the longest chain that includes the alcohol.
Snip the e off the suffix for the alkane and replace it with the suffix –ol, which stands for alcohol. For example, a two-carbon alcohol would not be ethane but ethanol.
-
Number the parent chain. Start numbering from the side closer to the hydroxyl group.
-
Identify all the substituents of the parent chain and name them.
-
Order the substituents alphabetically in front of the parent name.
-
Identify the location of the hydroxyl group by placing a number in front of the parent name.
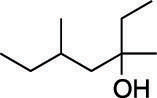
First, find the parent chain, as shown in the next figure. The parent chain is the longest chain of carbons that contains the hydroxyl group. In this case, the parent chain is seven carbons long, so this is a heptanol.
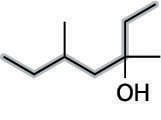
Then number the parent chain, starting from the end that reaches the hydroxyl group sooner. In this case, that's from right to left (see the next figure).
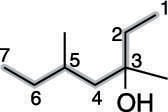
Find and name the substituents. The molecule shown in the next figure has two methyl group substituents — one at the number-three carbon and one at the number-five carbon.
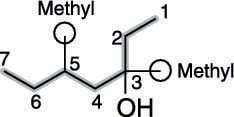
Then place the substituents (in alphabetical order) in front of the parent group. Indicate the position of the hydroxyl group by placing a number in front of the parent name. The two methyl groups combine to make dimethyl, and the name for this alcohol is, therefore 3,5-dimethyl-3-heptanol.

