In 1923, the physicist Louis de Broglie suggested that not only did waves exhibit particle-like aspects, but that the reverse was also true — all material particles should display wave-like properties.
How does this work? For a photon, momentum
And the wave vector, k, is equal to
De Broglie presented these apparently surprising suggestions in his Ph.D. thesis. Researchers put these suggestions to the test by sending a beam through a dual-slit apparatus to see whether the electron beam would act like it was made up of particles or waves. In the figure, you can see the setup and the results.
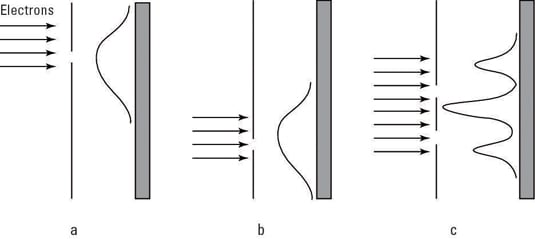
In Figure a, you can see a beam of electrons passing through a single slit and the resulting pattern on a screen. In Figure b, the electrons are passing through a second slit. Classically, you’d expect the intensities of Figures a and b simply to add when both slits are open:
I = I1 + I2
But that’s not what happened. What actually appeared was an interference pattern when both slits were open (Figure c), not just a sum of the two slits’ electron intensities.
The result was a validation of de Broglie’s invention of matter waves. Experiment bore out the relation that
and de Broglie was a success.
The existence of matter waves says that you add the waves’ amplitude,
not their intensities, to sum them:
You square the amplitude to get the intensity, and the phase difference between
is what actually creates the interference pattern that’s observed.

