If you imagine the microbial cell as a small factory, then the enzymes are the robots doing all the work. Enzymes are special proteins that are very good at converting things from one form to another. They do this by kicking off the chemical reactions needed for the conversion. The kinds of enzymes a microbe makes determine what type of metabolism the microbe will use to harness energy and grow.
Chemical reactions are at the heart of all cellular processes. Although you can’t see them happening, chemical reactions power all living things and many forces of nature. In science, these chemical reactions are written as equations with substances going into the reaction (the substrates) on one side of an arrow and the results coming out of the reaction (the products) on the other side of the arrow, like this:
Not all chemical reactions have the same number of substrates and products as the example, but they always have substrates going in and products coming out.
Chemical reactions are always accompanied by a change in energy. The substrates and products contain different amounts of energy. The difference between the amount of energy contained within the substrates and the amount of energy contained within the products determines whether energy needs to be put in or is released during the reaction.
Reactions that consume energy are called endergonic; reactions that release energy are called exergonic. The energy released from an exergonic reaction can either be stored for later use or be used right away to power an endergonic reaction.
Biologically important chemical reactions are needed for every process in the cell, but by themselves, the substrates in the cell would react so slowly that life would grind to a halt. Catalysts keep chemical reactions moving along quickly.
A catalyst is a protein (or sometimes an RNA) molecule that speeds up the rate of a chemical reaction without interfering in the reaction in any other way.
In the cell, these catalysts are called enzymes and they work by bringing substrates into close proximity with one another and by bending them into the right shape so they can react with one another and form products. Enzymes are usually much bigger than the substrates and the products of the reaction they catalyze.
Enzymes have a pocket specifically for the substrates and the products to fit inside of, called an active site. Lysozyme is much bigger than its substrate, peptidoglycan, which fits into its active site and is cleaved (split) into two sugars.
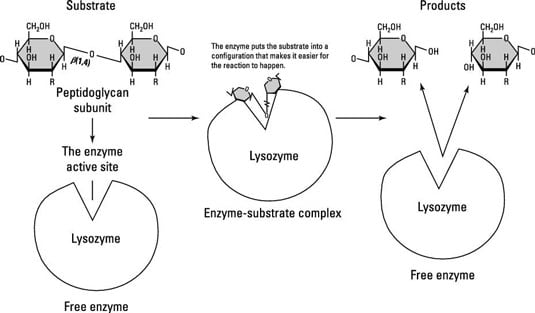
Enzymes are usually specific to one reaction or one group of similar reactions. Another interesting thing about enzymes and the reactions they catalyze is that most are reversible. That means that the products made in a reaction can recombine to re-form the substrates with the help of the same enzyme, as long as the energy change is reversed as well.
If the reaction produces or requires a lot of energy, there is usually a different enzyme for each direction of the reaction.
Coenzymes are small molecules that associate with an enzyme and help in its function but aren’t a substrate. NAD+/NADH is an example of a coenzyme that can associate with many different enzymes and helps with electron transfer. Prosthetic groups, on the other hand, are small molecules that bind permanently and are essential to the enzyme’s activity.

