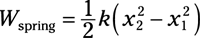Thermodynamics is filled with equations and formulas. Here’s a list of the most important ones you need to do the calculations necessary for solving thermodynamics problems.
Combustion equations:
Air-fuel ratio:
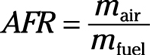
Hydrocarbon fuel combustion reaction:
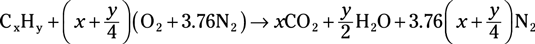
Compressibility calculations:
Compressibility factor Z: Pv = ZRT
Reduced temperature:
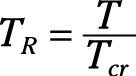
Reduced pressure:
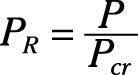
Pseudo-reduced specific volume:
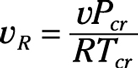
Efficiency equations:
Thermal efficiency:
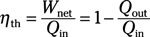
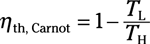
Coefficient of performance (refrigerator):
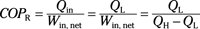
Coefficient of performance (heat pump):
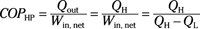
Energy equations:
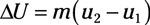
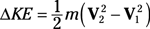
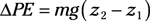
Entropy equations:
Entropy change for ideal gas, constant specific heat:
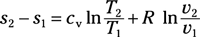
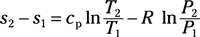
Entropy change for ideal gas, variable specific heat:
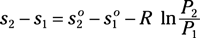
Irreversibility for a process:
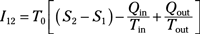
Ideal-gas formulas:
Ideal-gas law: Pv = RT
Gas constant:
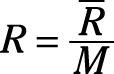
Ratio of specific heats:
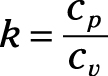
Isentropic process for ideal gas:
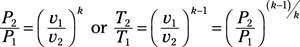
Moist air properties:
Relative humidity:
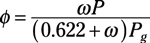
Specific humidity:
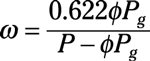
Properties of mixtures:
Quality liquid-vapor mixture:
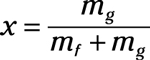
Saturated mixture property, y: y = yf + x · yfg
Work calculations:
Isobaric process: Wb = P0(V2 – V1)
Polytropic process:
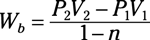
Isothermal process of an ideal gas:
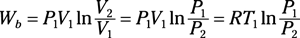
Shaft power:
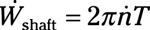
Spring work: